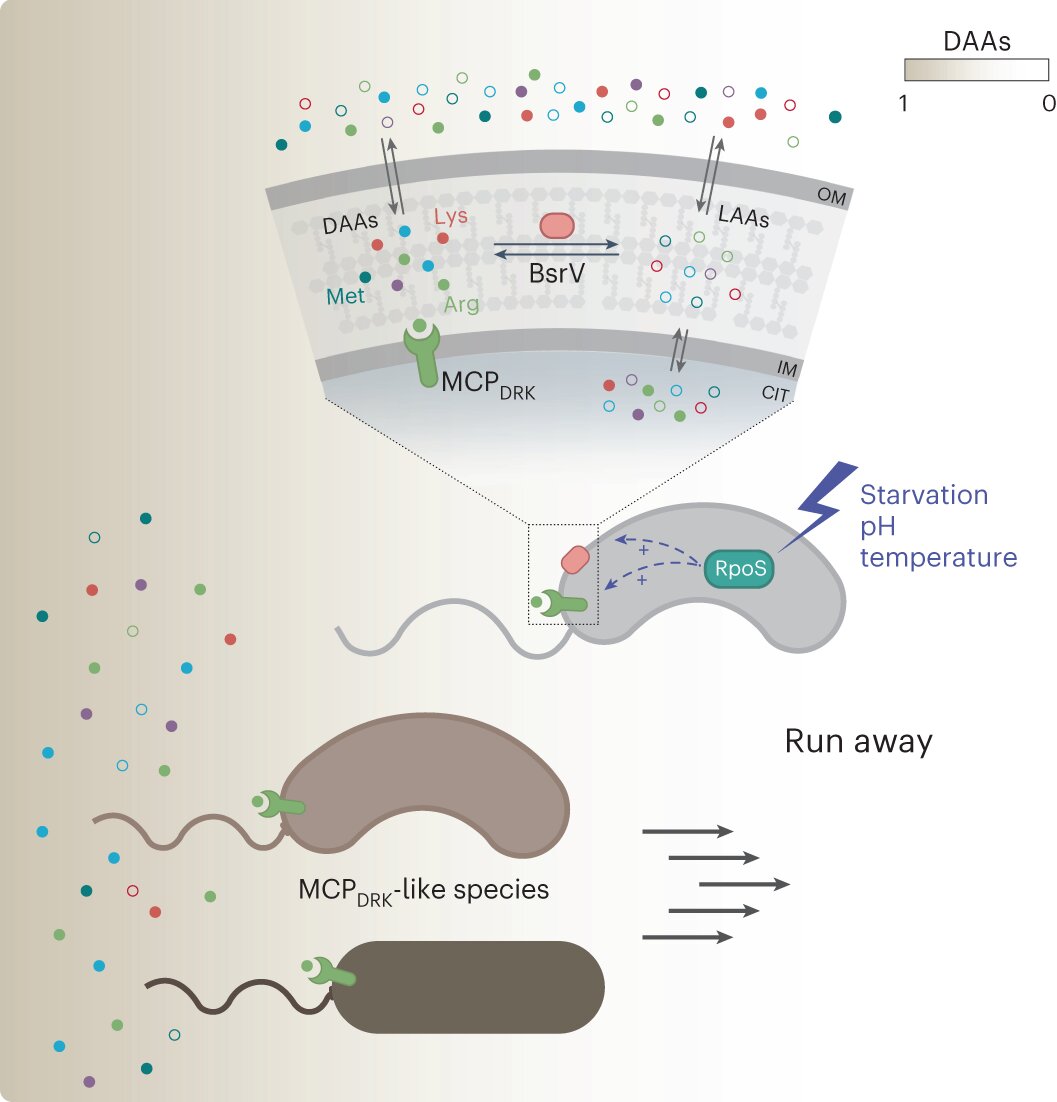
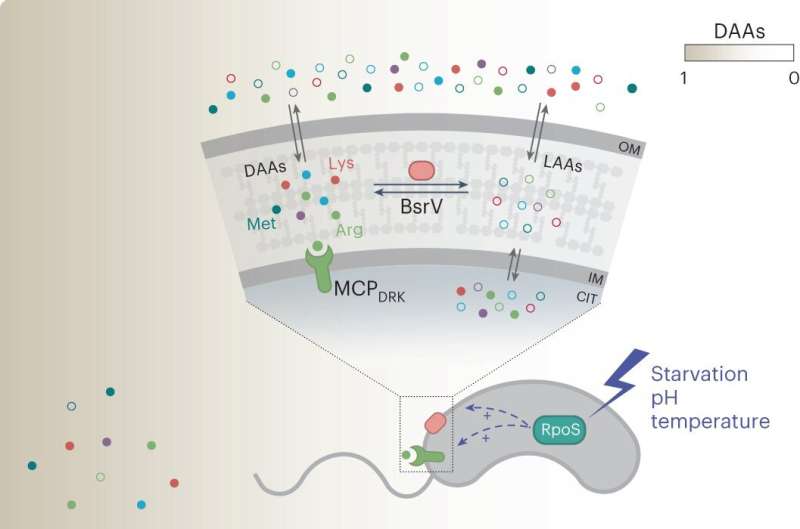
 Under certain environmental stresses (that is, starvation), the RpoS response induces the expression of both the broad-spectrum racemase BsrV and the dedicated d-Arg/d-Lys chemoreceptor MCPDRK. Among the d-amino acids (DAAs) produced by BsrV, d-Arg and d-Lys stand out as warning signals sensed by V. cholerae as well as other MCPDRK-encoding species. Chemotactic run-away response to these d-amino acids enables these bacterial communities to move away and explore more favorable niches. Credit: Nature Microbiology (2023). DOI: 10.1038/s41564-023-01419-6 Cholera bacteria use specific D-amino acids to escape unfavorable niches and form complex ecological systems. This is shown by a study led by a research group at Umeå University, Sweden. The discovery may eventually have significance for research into bacterial infections. The research is published in the journal Nature Microbiology.
Under certain environmental stresses (that is, starvation), the RpoS response induces the expression of both the broad-spectrum racemase BsrV and the dedicated d-Arg/d-Lys chemoreceptor MCPDRK. Among the d-amino acids (DAAs) produced by BsrV, d-Arg and d-Lys stand out as warning signals sensed by V. cholerae as well as other MCPDRK-encoding species. Chemotactic run-away response to these d-amino acids enables these bacterial communities to move away and explore more favorable niches. Credit: Nature Microbiology (2023). DOI: 10.1038/s41564-023-01419-6 Cholera bacteria use specific D-amino acids to escape unfavorable niches and form complex ecological systems. This is shown by a study led by a research group at Umeå University, Sweden. The discovery may eventually have significance for research into bacterial infections. The research is published in the journal Nature Microbiology.
“These findings deepen our knowledge of bacterial behavior and may impact the development of strategies to manipulate bacterial populations or control bacterial infections,” says Felipe Cava, professor at the Department of Molecular Biology at Umeå University.
The results unveiled a new role for D-amino acids in stress-driven bacterial chemotaxis. Using state-of-the-art technologies the researchers identified a previously uncharacterized chemoreceptor, MCPDRK, specific for D-Arginine and D-Lysine. The dual function of these D-amino acids as toxic-compounds and stress signals suggests a role in shaping microbial communities and influencing niche selection.
Chemotaxis, the ability of bacteria to respond to environmental cues and navigate their surroundings, is a widespread phenomenon that remains partially understood. While the core components of the chemotaxis pathway are conserved across bacteria, the specific signals that trigger chemotactic responses remain largely unidentified. This makes it hard to unravel the underlying factors that dictate bacterial navigation towards favorable environments or their evasion of detrimental conditions.
While the role of L-amino acids as signaling molecules has been extensively investigated, the precise function of their D-form counterparts in chemotaxis remains largely unknown. These intriguing molecules, generated from L-amino acids by racemase enzymes, play diverse and specific roles in various cellular processes, including cell wall formation, biofilm stability, spore germination, and interbacterial interactions.
Vibrio cholerae, the marine bacterium that causes the acute diarrheal disease Cholera, releases high concentrations of D-amino acids into the environment. However, their specific role in bacterial behavior remains underexplored. In this study, the researchers found that a mutant that did not produce extracellular D-amino acids exhibited reduced swimming capacity. A deeper look pointed D-Arginine and D-Lysine as repulsive chemotactic signals.
“This bacterium has a very sophisticated chemotaxis system which includes at least 45 chemoreceptors. Therefore, we strategically employed two-dimensional thermal proteome profiling instead of commonly used mutagenesis approaches. This strategy allowed us to effectively screen and identify the specific chemoreceptor responsible for D-Arginine and D-Lysine sensing, which we named MCPDRK,” says Oihane Irazoki at MIMS, The Laboratory for Molecular Infection Medicine Sweden at Umeå University and first author on the study.
The structural characterization of the sensory protein in complex with both D-amino acids allowed the identification of the key ligand-binding residues and prediction of functional orthologues in other species.
“Although our study primarily focuses on V. cholerae, MCPDRK is conserved among several species but its specificity for D-Arginine and D-Lysine seems to be restricted to those receptors that are transcriptionally linked to broad-spectrum racemases. Therefore, the applicability of these findings to other bacterial species needs additional investigation,” says Oihane Irazoki.
A key finding of the study is the multifaceted role of D-Arginine in shaping the biodiversity and structural dynamics of microbial communities. On one hand, it functions as a mechanism for clearing the environment of potential competitors, while on the other, it orchestrates the migration of the community towards more favorable ecological niches.
V. cholerae has evolved a “fight and flight” strategy, wherein the genes encoding the D-Arginine chemoreceptor and the broad-spectrum racemase responsible for D-amino acid production are positioned in tandem within a single operon. More interestingly, this arrangement is controlled by the stress sigma factor RpoS. Through the synchronized production of D-Arginine and its corresponding MCP, V. cholerae establishes a highly effective stress-responsive mechanism, while simultaneously preventing its futile activation in favorable conditions.
“An obvious question for the future will be how bacteria integrate and contextualize D-amino acid signaling as part of a bewildering network of environmental chemotactic cues and receptors. Understanding this decision-making will provide deeper insights into the ecological importance of D-amino acids in enabling bacterial adaptation to stress,” concludes professor Felipe Cava.
More information: Oihane Irazoki et al, d-amino acids signal a stress-dependent run-away response in Vibrio cholerae, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01419-6
Citation: D-amino acids play a role in stress-induced response in cholera bacterium (2023, June 27) retrieved 16 July 2024 from https://phys.org/news/2023-06-d-amino-acids-play-role-stress-induced.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.